What system has boundaries that allow both mass and energy to readily enter and leave?
Main Difference – Open vs Closed Organization
Thermodynamics is a branch of physics which explains the energy transfer betwixt objects and surrounding. Terms in thermodynamic can as well be used to understand chemical behavior of chemic species. Arrangement and surrounding are two basic terms used in thermodynamics. A system is a function of the universe which is existence studied and surrounding is the residue of the universe other than that particular system. The margin of the organisation which separates it from the surrounding is called boundary. Systems can exist in three means as open systems, closed systems, and isolated systems. The primary deviation between open up and closed system is that in an open organisation, matter can be exchanged with the surrounding whereas, in a closed system, matter cannot exist exchanged with the surrounding.
Key Areas Covered
1. What is an Open Organisation
– Definition, Characteristics
2. What is a Airtight System
– Definition, Characteristics
three. What is the deviation between Open and Airtight Systems
– Comparing of Cardinal Differences
Key Terms: Energy, Kinetic Energy System, Matter, Potential Energy, Surrounding, Thermodynamics
What is an Open System
An open organization tin be defined as a system which can exchange both matter and energy with the surrounding. For instance, the earth can exist recognized every bit an open organization. In this case, the world is the system, and space is the surrounding. Sunlight can accomplish the world surface and we tin send rockets to space. Sunlight and rocket tin be explained as energy and affair, respectively.
Commutation of matter between the open system and the surrounding occurs easily. This can also be easily explained past calculation matter or removing matter. But energy commutation is a chip more complicated considering energy can be exchanged in different forms and different conversions may occur during this exchange. Free energy is exchanged every bit estrus or any other form.
In thermodynamic terms, free energy exchange is characterized by potential energy, kinetic energy, and thermal energy. Potential energy is the stored energy. Kinetic energy is the energy carrying by an object while moving. However, the energy of a system e'er exists in one of these three states or in two states at the aforementioned time. For example, a stationary object can substitution oestrus with the surrounding. Then it has both potential free energy and thermal free energy. Energy can be exchanged or transferred as potential energy or kinetic energy. Merely sometimes, potential energy can be converted into kinetic energy or the reverse can occur. Thermal energy or estrus is as well exchanged between open systems and their surround.
Due to the capability of exchanging matter between open up organization and surrounding, the internal mass of an open up system varies with time. If affair is added, the mass will increase and if matter is removed, the mass will decrease.

Effigy 1: Since the mug is not covered, both free energy and affair tin can be exchanged with the surrounding. Thus, this is an open system.
What is a Closed System
A closed system is a system where simply energy tin can be exchanged but not matter. Thing cannot exist exchanged in a closed organisation because matter contains particles which cannot cross the purlieus of the system. Only energy is passed through this boundary every bit photons because energy is not particulate. Therefore, in a closed system, the mass remains constant because the affair cannot be removed or added. But free energy can be transferred mostly as heat or thermal energy.
For example, if a warm cup of water is covered by placing a chapeau on the top of the cup, and then steam cannot escape the system considering of the hat. The gas molecules in the air also cannot enter the cup because of the lid. And then, in that location is no exchange of matter. But if we touch the lid subsequently some time, we tin feel that it is warm. The cup too will experience warm; this indicates that energy is coming outside every bit thermal energy. If this organization is kept at a normal temperature for a long time, information technology can be observed that the loving cup, lid or h2o is no longer warm. This is because the system has shared rut free energy with the surrounding until the temperature of the system becomes equal to the temperature of the surrounding. This is chosen an equilibrium.

Figure ii: The covered pot is an example of a closed organisation since it cannot exchange thing with the surrounding considering of the hat.
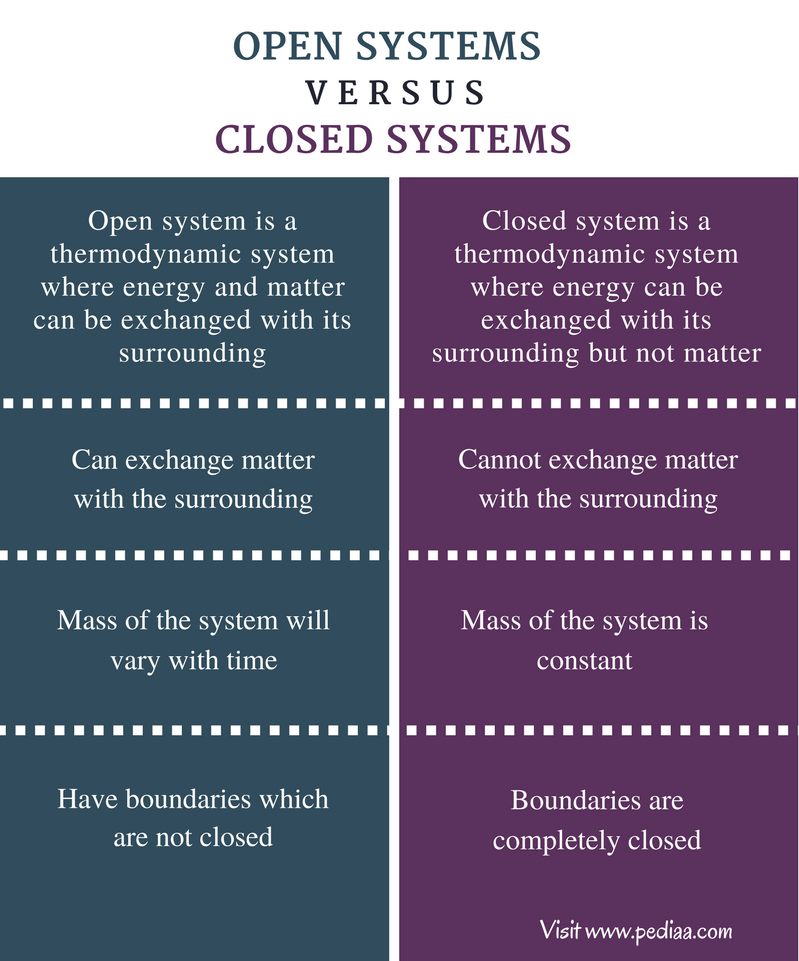
Difference Betwixt Open and Closed Systems
Definition
Open Organization:An open system is a thermodynamic system where energy and matter can be exchanged with its surrounding.
Airtight System: A closed organisation is a thermodynamic organisation where energy can be exchanged with its surrounding but not matter.
Exchange of Affair
Open System: Open systems can exchange matter with the surrounding.
Closed System: Closed systems cannot exchange matter with the surrounding.
Internal Mass
Open up System: The mass of the organization will vary with time in open systems.
Airtight Organisation: In closed systems, the mass of the system is abiding.
Boundary of the System
Open up System: Open systems have boundaries which are not airtight.
Closed System: The boundary of a airtight system is completely closed.
Conclusion
Everywhere in the environs, there are interactions between systems and their surroundings. Systems tin can be either opened, closed or isolated. The main difference between open and airtight system is that, in open up system, affair can be exchanged with the surrounding whereas, in a closed organisation, thing cannot be exchanged with the surrounding.
References:
1."A System and Its Environment." Chemical science LibreTexts. Libretexts, 21 July 2016. Spider web. Available hither. 16 June 2017.
2."Open up, Closed and Isolated Systems in Concrete Chemical science." Foundations of Concrete Science. Northward.p., north.d. Web. Available hither. sixteen June 2017.
Image Courtesy:
i. "345707" (Public Domain) via Pixabay
2. "coffee steam ii" by waferboard (CC By two.0) via Flickr

Source: https://pediaa.com/difference-between-open-and-closed-system/
Post a Comment for "What system has boundaries that allow both mass and energy to readily enter and leave?"